Research Summary | ||
|
Human behavior is a reflection of brain function. Our emotions, our intelligence, and our ability to learn and remember all depend on the complexity of connections between hundreds of billions of nerve cells in the human brain. These intricate connections form neuronal circuits that are constantly modified during life by experience. Neurons connect with each other at specialized areas, called synapses, where signals are sent and received between neurons. At synapses, active neurons release neurotransmitters that travel across the narrow gap between the neurons and bind to specific receptor molecules on the neighboring neuron (Figure 1). Each of the billions of neurons in the human brain can have up to 10,000 synaptic connections. By establishing an ever-changing network of synapses, the brain is able to attain the level of functional complexity that underlies human behavior. The formation and withdrawal of synaptic connections between neurons is a dynamic process that can be modified by experience. In addition, experience can change the efficacy of existing synapses. This constant change in the synaptic communication between neurons is called synaptic plasticity and is critical for higher brain functions such as learning and memory. We are interested in the mechanisms that regulate synaptic transmission and synaptic plasticity. Our general approach is to study molecular and cellular mechanisms that regulate neurotransmitter receptors and synapse function. |
Click here to see the bigger size show |
|
|
Neurotransmitter receptors mediate the response of neurons to neurotransmitters released at synapses and are a central convergence point for transmission of signals between neurons. Modulation of the function of these receptors is a powerful and efficient way to modulate synaptic communication and synaptic plasticity. Studies from our laboratory and several other laboratories over the last 15 years have shown that the dynamic regulation of receptor function and expression at synapses mediates many forms of synaptic plasticity in the brain. We have recently focused on the mechanisms that underlie the regulation of glutamate receptors, the major excitatory neurotransmitter receptors in the brain. These receptors are neurotransmitter-dependent ion channels that allow ions to pass through the neuronal cell membrane, resulting in the excitation of the neuron. Glutamate receptors play critical roles in learning and memory, development of the brain, and neurological and psychiatric disorders. |
||
Phosporylation of Neurotransmitter Receptors:
Our previous studies have shown that the function of neurotransmitter receptors can be highly regulated by several cellular mechanisms, including a process called protein phosphorylation. This process occurs when protein kinase enzymes catalyze the chemical transfer of phosphate molecules from ATP to a specific substrate protein such as a receptor molecule. The addition of the negatively charged phosphate group alters the structure of the receptor and can regulate its functional properties. Protein phosphorylation is reversible; enzymes called protein phosphatases can remove the phosphate group from the receptor. Protein phosphorylation and dephosphorylation are highly regulated processes, and thus the level of receptor phosphorylation in a neuron is constantly modified by neurotransmitters and hormones released by the surrounding neurons.
Recently we have focused on the role of protein phosphorylation of glutamate receptors in mediating synaptic plasticity. Glutamate receptors consist of a complex of four protein subunits that traverse the neuronal cell membrane and contain the ion channel and the binding site for the neurotransmitter (Figure 2). These receptors come in many different subtypes and are classified based on their pharmacological and physiological properties into N-methyl-D-aspartate (NMDA) and α-amino-3-hydroxy-5-methyl-4-isoxazole-propionate (AMPA) receptors. We have found that protein phosphorylation of these receptors is complex. Individual subunits can be multiply phosphorylated by several protein kinases and dephosphorylated by a variety of protein phosphatases. Each of these phosphorylation events can regulate the receptors in distinct ways. For example, phosphorylation of the GluR1 subunit of the AMPA receptor potentiates the responsiveness of the receptor to glutamate and also increases the number of receptors at synapses, thus enhancing the efficiency of synaptic transmission. In contrast, phosphorylation of the GluR2 subunit appears to decrease the number of AMPA receptors at synapses and thus decreases the strength of synaptic transmission. We have investigated whether the phosphorylation of glutamate receptors is involved in the mechanisms underlying well-characterized cellular models of learning and memory called long-term potentiation (LTP) and long-term depression (LTD). LTP and LTD are processes in the brain in which the communication between neurons can be rapidly modified by surrounding neuronal activity to produce long-lasting changes in the efficiency of synaptic transmission. It is these long-lasting changes in neuronal connections that form specific neuronal circuits that encode memories. By generating mutant mice that specifically lack the phosphorylation sites on the GluR1 subunit, we have shown that GluR1 phosphorylation is important for LTP and LTD in the hippocampus, a region of the brain important for learning and memory. Moreover, these mutant mice have problems remembering spatial learning tasks, demonstrating that GluR1 phosphorylation is important for memory retention. In addition, by generating mutant mice that specifically lack a phosphorylation site on the GluR2 subunit, we have recently shown that GluR2 phosphorylation is critical for LTD in the cerebellum, a region of the brain involved in motor learning. Future work will test whether GluR2 phosphorylation is important for motor-learning paradigms such as associative eyeblink conditioning. In a recent study, in collaboration with Robert Malinow (Cold Spring Harbor Laboratory), we have found that phosphorylation of GluR1 is also critical for the formation of emotional memories. Heightened states of emotion, such as fear or stress, induce the release of neurotransmitters, such as norepinephrine, that can make events more memorable. We found that during these emotional states norepinephrine increases the phosphorylation of GluR1 and primes the receptor to be added to synapses. This enhances the strengthening of synaptic connections and memory formation. Remarkably, norepinephrine no longer has this priming effect in mutant mice that lack the GluR1 phosphorylation sites, indicating that GluR1 phosphorylation is required for this process. |
||
Synaptic Targeting of Neurotransmitter Receptors:
The presence of high concentrations of receptors in the cell membrane at synaptic connections is critical for the efficient transmission of signals between neurons. Neurotransmitter receptors are specifically transported within the cell from the cell body to synapses and are retained at synapses by a complex synaptic scaffold of interacting proteins (Figure 3).
The molecular mechanisms underlying the initial formation and long-term maintenance of receptor clustering at synapses are not clear. We are interested in identifying factors that initiate the development of synapses and have been searching for factors that are released from the presynaptic nerve terminal and induce clustering of neurotransmitter receptors at synapses. Recently we have reconstituted artificial synapses between neurons and nonneuronal cells as a simple model system to investigate the molecular components required for recruitment of AMPA receptors to synapses. Using this system, we found that axons specifically express factors that recruit the AMPA receptors to sites of contact between axons and nonneuronal cells artificially expressing AMPA receptors. In addition, we found that axonally derived secreted proteins called neuronal pentraxins directly bind AMPA receptors and are required for AMPA receptor recruitment to these artificial synapses. Moreover, neuronal pentraxins are also required for the synaptic targeting of AMPA receptor subtypes to neuron-neuron synapses in the brain. These results indicate that neuronal pentraxins serve as critical trans-synaptic factors for AMPA receptor recruitment to synapses. |
|
|
|
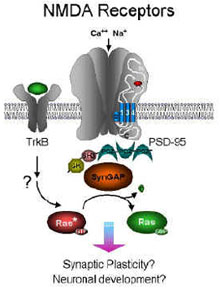
Recently it has become clear that the synaptic targeting of AMPA receptors is a dynamic process and that the level of receptors at synapses can be rapidly regulated to affect the strength of synaptic connections. The regulation of this synaptic targeting can modulate the number of receptors at synapses and may be important for synaptic plasticity. We have been examining the molecular mechanisms that dynamically regulate the targeting of glutamate receptors to synapses. We identified a variety of proteins that directly or indirectly bind to the intracellular regions of receptors (Figure 4) and help to escort or retain the receptors in different parts of the cell. We identified glutamate receptor–interacting proteins (GRIPs), which are a family of neuronal proteins that bind directly to AMPA receptors and appear to be important in the transport of these receptors to synapses. The GRIPs act as molecular Velcro to link AMPA receptors to other neuronal proteins involved in the regulation of AMPA receptor function. In addition, we have found that AMPA receptor subunits also interact with PICK1, a protein kinase C–binding protein that is found at excitatory synapses. AMPA receptors also interact with many other proteins including the the NSF protein, a protein involved in the regulation of membrane fusion events, and the 4.1N protein, a protein that interacts with actin cytoskeleton. The AMPA receptor–interacting proteins are involved in the proper subcellular trafficking and synaptic targeting of the receptors. In recent studies we have shown that PICK1 and GRIPs are required for cerebellar LTD. Deletion of the genes encoding PICK1 or the GRIPs in mice eliminates cerebellar LTD. We have also found that PICK1 and GRIPs also play important roles in other forms of synaptic plasticity. Recent studies have characterized a novel form of plasticity that we call CARP (Calcium permeable AMPA Receptor Plasticity) that involves the dynamic exchange of different subtypes of AMPA receptors. In this form of plasticity calcium impermeable AMPA receptors and calcium permeable AMPA receptors are dynamically exchanged. Interestingly, PICK1 and phosphorylation of GluR2 are also critical for these forms of plasticity. These results indicate that modulation of the physical interaction of GRIP, PICK1, and other proteins with AMPA receptors may be a major mechanism in the regulation of receptor function, synaptic transmission, and synaptic plasticity. Recent human genetic studies have also linked the PICK1 gene to schizophrenia. In addition to these studies on AMPA receptor–interacting proteins, we have been characterizing a separate NMDA receptor–associated protein complex that is involved in synaptic targeting and downstream signaling of NMDA receptors. We have identified a novel excitatory synapse-specific Ras-GTPase–activating protein, called synGAP, that is associated with the NMDA receptor and regulates a specific synaptic Ras signaling pathway. Mutation of the synGAP gene disrupts LTP in the hippocampus as well as spatial learning, demonstrating the importance of synGAP signaling for learning and memory. The synGAP gene has also been linked to schizophrenia in human genetics studies. |
|
|
|
In summary, our laboratory has been investigating the molecular mechanisms in the regulation of neurotransmitter receptor function and synaptic transmission. Our studies of neurotransmitter receptors, including the major excitatory receptors in the brain, the glutamate receptors, demonstrate that protein phosphorylation of receptors is a major mechanism for the control of their function and is critical for the regulation of synaptic communication and learning and memory. Moreover, recent studies on the regulation of the synaptic localization of receptors have shown that receptor-associated proteins—such as the neuronal pentraxins, GRIP, PICK1, NSF, 4.1N and synGAP—are critical modulators of receptor synaptic targeting and synaptic plasticity. These studies demonstrate that the regulation of neurotransmitter receptor function plays a central role in the modulation of synaptic transmission and may be an important determinant in brain function and human behavior. |
||

